From the moisturizer in your skincare routine to the sweetener in your favorite foods, glycerine plays a crucial role in countless products you use every day. For example, glycerine is commonly used in hand creams to lock in moisture and improve skin softness, demonstrating its versatility and several benefits in both household and personal care products. This versatile compound, also called glycerol or glycerin, has been quietly revolutionizing industries for over two centuries since its accidental discovery in 1779.
Whether you’re a skincare enthusiast seeking natural ingredients, a food industry professional exploring safe additives, or simply curious about the science behind everyday products, this comprehensive guide covers everything you need to know about glycerine. We’ll explore its chemical properties, production methods, diverse applications, and the remarkable benefits that make it an essential ingredient in modern manufacturing.
What is Glycerine?
Glycerine, also known as glycerol, is a colorless, odorless, sweet-tasting viscous liquid classified as a sugar alcohol or polyol. Its chemical formula is C₃H₈O₃, with the IUPAC name 1,2,3-propanetriol, reflecting its three-carbon backbone and three hydroxyl (-OH) groups. The correct chemical name, glycerol, is often used interchangeably with glycerine or glycerin in scientific and industrial contexts, and the name glycerol highlights its classification as a polyol. This syrupy liquid has a molecular weight of 92.09 g/mol and serves as a fundamental building block in both natural and synthetic chemistry.
Naturally, glycerine forms the backbone of triglycerides found in animal sources, such as animal fats, and vegetable oils such as olive oil and coconut oil. Glycerine is commonly found in a wide range of natural and commercial products. When consumed, glycerine undergoes metabolism in the body, converting into energy substrates that support liver function and cellular energy production. Glycerol metabolism primarily occurs in the liver, where it plays a crucial role in energy production and lipid synthesis.

This metabolic pathway is significant for maintaining glucose levels and is particularly relevant to health conditions such as liver disease, diabetes, and metabolic syndrome. During fat breakdown, glycerol is released from adipose tissue and utilized in metabolic pathways such as glycolysis and gluconeogenesis. The name glycerine comes from the Greek word “glukus,” meaning sweet, coined in 1811 by chemist Michel Eugène Chevreul, reflecting its characteristic sweet taste, approximately 60% as sweet as table sugar.
Key features of glycerine include its hygroscopic nature (absorbing moisture from the air), non-toxic profile, and liquid state over a wide temperature range. Glycerine is completely water-soluble, making it miscible with water in any proportion and forming aqueous solutions widely used in various industries.
Chemical Properties and Structure
The physical properties of glycerine, such as its melting point, boiling point, and viscosity, are central to its performance in various applications. Glycerine’s unique chemical composition underpins its versatility. As a triol sugar alcohol, it is classified as one of the organic compounds due to its carbon-based structure, and it exhibits a high boiling point of 290°C (554°F) due to extensive hydrogen bonding between molecules. Each glycerine molecule can form multiple hydrogen bonds, contributing to its viscosity and thermal stability.
Its solvent properties, with an XLogP3 value of -1.8, indicate high water affinity, enabling it to dissolve substances that resist water alone. When mixed with water, glycerine forms a solution with notable characteristics. The combination of glycerine and water creates a homogeneous mixture, commonly referred to as a glycerol-water mixture, which is valued in chemical and industrial contexts for its stability, tunable viscosity, and ability to modify freezing and boiling points. Glycerine’s ability to depress freezing temperatures (e.g., a 70% glycerol-water solution freezes near -38°C) makes it useful in antifreeze applications.
Physical Properties of Glycerine
Glycerine, also called glycerol or 1,2,3-propanetriol, is renowned for its unique combination of physical properties that make it indispensable across industries. In its pure form, glycerine is a colorless, odorless, and syrupy liquid with a distinctly sweet taste, attributes that contribute to its popularity in skin care, food, and pharmaceutical formulations. As a sugar alcohol, glycerine’s chemical composition (C₃H₈O₃) features three hydroxyl groups, giving it exceptional water solubility and the ability to form stable aqueous solutions.
One of glycerine’s standout characteristics is its high boiling point of 290°C (554°F), which allows it to remain stable under heat and makes it suitable for processes requiring elevated temperatures. Its viscosity and hygroscopic nature enable it to retain moisture, a property highly valued in products designed to combat dry skin and maintain skin health. While refined glycerine is typically a clear, colorless liquid, crude forms, often a byproduct of biodiesel production, may appear as a brown colored liquid due to the presence of other impurities and residual oils.

These physical properties not only enhance the sensory experience of products but also ensure that glycerine functions effectively as a humectant, solvent, and preservative. Whether derived from vegetable oils or animal sources, glycerine’s versatility as a water-soluble, non-irritating, and stable ingredient underpins its role as an essential ingredient in modern skin care, food, and pharmaceutical products.
Production Methods
Glycerine is produced via two main routes: natural extraction from biological sources and synthetic manufacture from petrochemicals. In natural production, triglycerides from fats and oils are hydrolyzed, broken down through hydrolysis reactions, often using sodium hydroxide or sodium carbonate to yield glycerol and fatty acids. Biodiesel production has increased crude glycerol availability as a byproduct of vegetable oil transesterification. During purification, residual salt, sodium chloride, and other impurities are removed to achieve high-purity glycerine suitable for industrial use.
Natural Production
Vegetable glycerine is derived from plant oils like palm, soy, and coconut. Oils undergo hydrolysis or saponification, breaking triglycerides into glycerol and fatty acids. Crude glycerol obtained contains impurities such as methanol and soap residues and requires purification. The Soap and Detergent Association sets standards to ensure quality.
Synthetic Production
Synthetic glycerine is made from propylene via chlorination to allyl chloride, oxidation to epichlorohydrin, and hydrolysis to glycerine. This route produces high-purity pharmaceutical-grade glycerine. Purification involves vacuum distillation, ion exchange, and activated carbon treatment to remove impurities and ensure safety.
Major Applications and Uses
Glycerine’s safety, solvent abilities, moisture retention, and stability have led to its use across industries, including the pharmaceutical industry, where its moisturizing properties are highly valued. In industrial applications, glycerine is often used in combination with other chemicals to enhance formulations and performance.
Personal Care and Cosmetics
Glycerine is a key humectant in skincare, drawing moisture to the skin’s surface and improving hydration for all skin types. It softens and soothes skin, contributing antibacterial and antifungal properties. The health benefits of glycerine include its antibacterial, antifungal, and antiviral effects, which enhance its role as a preservative and make it a highly beneficial ingredient in skin care products. For those with sensitive or acne-prone skin, glycerine helps prevent further irritation by providing gentle hydration without clogging pores. Concentrations of 5-15% are common in moisturizers.
In soap making, glycerine is either retained to create moisturizing soaps or extracted for cosmetics. Glycerine is also widely used in beauty products, including DIY cosmetics and cheek stains, where it helps moisturize and enhance appearance. Hair care products use glycerine to maintain moisture and manageability. Toothpaste formulations contain 20-30% glycerine to prevent drying and improve taste.
Food and Beverage Industry
Recognized as safe by the FDA, certain grades of glycerine are approved for human consumption according to regulatory standards. Glycerine functions as a sweetener (about 60% as sweet as sugar), preservative, humectant, and solvent in foods. It prevents crystallization in candies and baked goods and enhances texture and shelf life. In beverages, it carries flavors and improves mouthfeel.
Pharmaceutical Applications
Glycerine acts as a solvent for active ingredients, masking tastes in syrups and elixirs. It is used in suppositories as an osmotic laxative, producing bowel movements within 15-30 minutes by drawing water into the bowel to induce evacuation. In eye care and other medicinal uses, glycerine is often administered as a solution, benefiting from its lubricating and moisture-retaining properties.

Industrial Applications
Glycerine is a precursor to nitroglycerin, used in explosives and heart medications. Its antifreeze properties protect equipment in cold temperatures with lower toxicity than ethylene glycol. The boiling point and flammability of glycerine are often measured using the open cup method, which is important for safety and occupational health classifications. It serves as a cryoprotectant in laboratories and a damping fluid in pressure gauges.
Skin Care Benefits
Moisturizing Properties
Glycerine’s hygroscopic nature allows it to attract and retain moisture from the air and deeper skin layers, hydrating the stratum corneum and supporting the skin barrier. It outperforms many synthetic humectants like propylene glycol due to its smaller molecular size and water affinity. It also forms hydrogen bonds with skin proteins and lipids, reducing water loss and maintaining hydration. Anti-inflammatory effects help soothe eczema and dermatitis without irritation.
Anti-Aging Benefits
By maintaining skin plumpness and elasticity through hydration, glycerine reduces the appearance of wrinkles and fine lines. It promotes proper skin cell maturation and barrier function, aiding long-term skin health. Gentle exfoliation properties help renew skin without irritation.
Acne and Sensitive Skin
Glycerine is non-comedogenic, oil-free, and safe for acne-prone and sensitive skin. It hydrates without clogging pores and balances sebum production. Its gentle cleansing and healing properties support recovery from acne and skin irritations.
Glycerine in Hair Care
Glycerine’s moisture-attracting nature makes it essential for hydrating dry or chemically treated hair. It penetrates hair shafts to retain moisture, reduce breakage, and boost shine. When paired with propylene glycol, it helps balance hydration across different climates. Plant-derived vegetable glycerine supports the growing demand for natural, sustainable beauty ingredients. Its water solubility and mild preservative qualities enhance product stability and performance. Though safe and effective, extremely humid or dry conditions may require formulation tweaks to avoid frizz or dryness. Backed by industry standards and safety data, glycerine remains a trusted ingredient globally.
You may be interested in: Biotinoyl Tripeptide-1: The Advanced Biotin Derivative Revolutionizing Hair and Skin Care

Safety and Toxicity Profile
The Food and Drug Administration’s GRAS status confirms glycerine’s safety for ingestion and topical use, underscoring the agency’s regulatory oversight of permissible ingredients. Animal studies show very low acute toxicity with high LD50 values, and adverse effects are rare and generally mild. Inhalation safety is well established with workplace exposure limits set by NIOSH, and the NIOSH Pocket Guide provides authoritative information on exposure limits, chemical hazards, and recommended protective measures for glycerine.
CAMEO Chemicals is also a valuable free database for chemical safety and hazard information, offering comprehensive data and resources for chemical handling and safety management. Skin compatibility is excellent across populations, with rare allergic reactions generally due to impurities. Long-term use shows no cumulative toxicity.
Potential risks include excessive consumption, causing mild gastrointestinal symptoms, mainly in children. Historical contamination incidents led to strict quality controls, ensuring pharmaceutical-grade glycerine is free from harmful substances. In occupational settings, understanding chemical hazards and adhering to occupational safety standards set by organizations such as NIOSH and OSHA is essential for proper handling and worker protection.
How to Use Glycerine
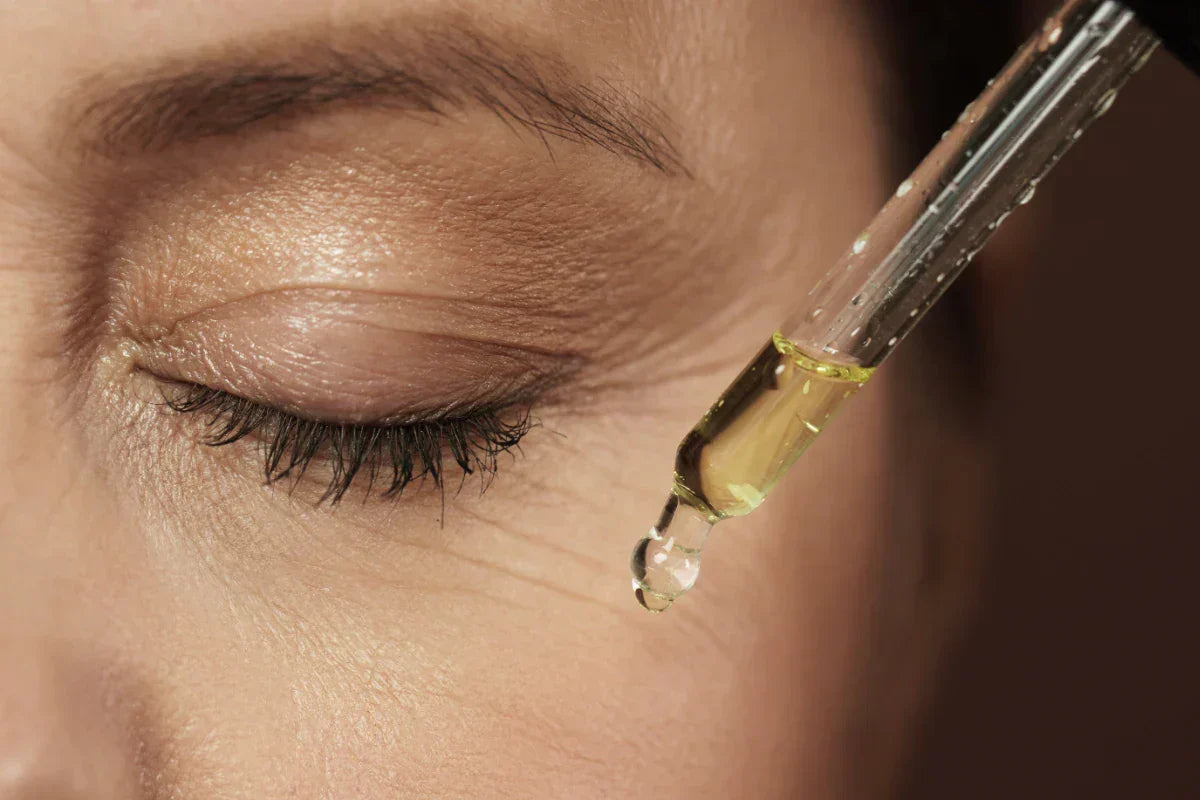
Glycerine is commonly diluted with water or other liquids to achieve desired concentrations for skincare or pharmaceutical applications. Pure glycerine should not be applied directly to the skin at high concentrations to avoid dryness. DIY skincare products often use 5-15% glycerine mixed with gentle surfactants or hydrating ingredients. Hydrating toners, moisturizing masks, and treatments for dry skin benefit from glycerine’s properties. In commercial products, look for glycerine listed among the main ingredients and choose USP-grade or certified organic options for sensitive skin.
Types and Grades of Glycerine
Purity Levels
- Crude glycerine (80-91% glycerol) is a byproduct of biodiesel production, requiring purification.
- Refined glycerine (86.5%) suits industrial uses.
- Technical grade (99.5%) is used in cosmetics and food.
- USP grade (99.7%+) meets pharmaceutical standards.
- Source-Based Classifications
- Vegetable glycerine from plant oils appeals to natural product markets.
- Synthetic glycerine offers consistent purity.
- Organic, non-GMO, kosher, halal, and fair trade certifications address consumer preferences.
Regulatory Information on Glycerine
Glycerine’s widespread use in skin care, food, and cosmetics is supported by rigorous regulatory oversight from leading authorities worldwide. In the United States, the Food and Drug Administration (FDA) classifies glycerine as “generally recognized as safe” (GRAS) for use in food and cosmetic products, ensuring consumer safety and product integrity. The Soap and Detergent Association and Detergent Association set industry standards for purity and quality, particularly for applications in soap and personal care formulations.
Occupational safety is a top priority in the handling and manufacturing of chemicals like glycerine. The Occupational Safety and Health Administration (OSHA) and the National Institute for Occupational Safety and Health (NIOSH) provide comprehensive guidelines for safe workplace practices, including exposure limits and hazard communication. The NIOSH Pocket Guide and the CRC Handbook of Chemistry and Physics are authoritative resources for chemical properties, recommended handling procedures, and emergency measures.

In the European Union, the European Chemicals Agency (ECHA) regulates glycerine under the REACH framework, requiring thorough evaluation and registration to ensure environmental and human health protection. The Environmental Protection Agency (EPA) oversees glycerine’s use in biodiesel production, monitoring its environmental impact and safe disposal.
These regulatory frameworks guarantee that glycerine used in food, cosmetics, and skin care meets stringent safety and quality standards. For manufacturers and brands, compliance with these regulations is essential for market access and consumer trust, reinforcing glycerine’s reputation as a safe, reliable, and essential ingredient in a wide range of products.
Conclusion
Glycerine is a natural, versatile compound essential across many industries. Its safety, moisturizing abilities, and chemical properties make it invaluable in skincare, food, pharmaceuticals, and industrial applications. Whether derived from sustainable plant sources or synthesized chemically, glycerine remains a cornerstone ingredient with expanding uses. Understanding glycerine’s properties and applications empowers consumers and manufacturers to make informed choices, ensuring continued benefits from this remarkable compound in daily life and industry.
Frequently Asked Questions on Glycerine (FAQ)
1. What is glycerine used for?
Glycerine is used in skincare, food, and pharmaceuticals for its moisturizing and stabilizing properties. It helps keep products smooth, hydrated, and long-lasting.
2. Is glycerine safe for skin?
Yes, glycerine is safe, gentle, and suitable for all skin types. It hydrates skin without clogging pores or causing irritation.
3. Is glycerine natural or synthetic?
Glycerine can come from natural plant oils or be produced synthetically. Both forms are safe and widely used in cosmetics and food.
4. Can glycerine be used on hair?
Yes, glycerine helps hydrate dry or damaged hair by attracting and retaining moisture.
It also reduces frizz and improves shine when used in balanced formulations.
5. Is glycerine safe to eat?
Food-grade glycerine is approved by global authorities and is safe for consumption.
It is commonly used as a sweetener, humectant, and preservative in food products.